Webinar: The Business of Genomic Testing
![]() Presenter: James M. Crawford, MD, PhD, Executive Director and Senior Vice President, Laboratory Services at North Shore-LIJ Health System and Professor and Chair, Department of Pathology and Laboratory Medicine at Hofstra North Shore-LIJ School of Medicine
Presenter: James M. Crawford, MD, PhD, Executive Director and Senior Vice President, Laboratory Services at North Shore-LIJ Health System and Professor and Chair, Department of Pathology and Laboratory Medicine at Hofstra North Shore-LIJ School of Medicine
Date: October 30, 2014
Abstract
Integration of “genomic medicine” into routine medical care is rapidly increasing, given the association of genetic variation with disease risk, development and progression of somatic diseases and of cancer, and drug efficacy or risk of adverse events. The core technology for support of genomic medicine is “next-generation sequencing” (NGS), broadly defined as gene sequencing using massively parallel strategies.
The institutional decision to bring NGS and genomic medicine into the clinical armamentarium is challenging because the actual clinical evidence base for systematic use of this technology is not established.
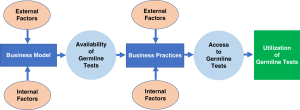
This webinar will discuss the findings of a College of American Pathologists survey of “early adopters” of NGS (Crawford et al.). Our study objective was to identify the reasons for health systems to bring next-generation sequencing into their clinical laboratories and to understand the process by which such decisions were made. A standardized open-ended interview was conducted with the laboratory medical directors and/or department of pathology chairs of 13 different academic institutions in 10 different states.
Genomic testing for cancer dominated the institutional decision making, with three primary reasons: more effective delivery of cancer care, the perceived need for institutional leadership in the field of genomics, and the premise that genomics will eventually be cost-effective. Barriers to implementation included implementation cost; the time and effort needed to maintain this newer testing; challenges in interpreting genetic variants; establishing the bioinformatics infrastructure; and curating data from medical, ethical, and legal standpoints. Ultimate success depended on alignment with institutional strengths and priorities and working closely with institutional clinical programs.
These early adopters uniformly viewed genomic analysis as an imperative for developing their expertise in the implementation and practice of genomic medicine.
A limitation of this study is that the respondents did not actually know the ultimate clinical utility of NGS testing, as regards its impact on patient outcomes and the efficiency of delivering clinical care. Nevertheless, by their deciding to proceed with NGS implementation, this study may help guide others who are contemplating bringing this technology into their institutions for the care of their patient populations.
Reference: Crawford JM, Bry L, Pfeifer J, Caughran SK, Black-Schaffer S, Kant JA, Kaufman JA. (2014) The business argument for genomic testing: a survey of early adopters. Genetics in Medicine 2014 Jul 10. doi: 10.1038/gim.2014.60. PMID 25010053 [Epub ahead of print]
About the Presenter
James M Crawford, MD, PhD, is Professor and Chair, Department of Pathology and Laboratory Medicine, Hofstra North Shore-LIJ School of Medicine, and Executive Director and Senior Vice President of Laboratory Services, North Shore-LIJ Health System. He received his MD and PhD degrees from Duke University School of Medicine (1983), and his post-graduate training in Anatomic Pathology and Gastrointestinal Pathology at Brigham and Women’s Hospital (1983-1987), followed by a Fellowship in Hepatic Pathology at the Royal Free Hospital in London (1989). He has served on faculty and as a staff pathologist at Harvard Medical School and Brigham and Women’s Hospital (1988-1996), and the Yale University School of Medicine (1997-1999). From 1999-2008, he was Professor and Chair, Department of Pathology, Immunology and Laboratory Medicine at the University of Florida College of Medicine in Gainesville, FL.
He has held his current position at North Shore-LIJ since January 2009. Dr. Crawford is a leading proponent of the role of Pathology and the Clinical Laboratory industry in patient centered care, and in accountable care. Since December 2008 he has served as co-chair of the PCPCC “Center for eHealth” (2008-2012) and “eHealth Special Interest Group” (2012-present). He is extensively involved with Pathology organizations as an advocate for patient centered- and accountable care; these include the College of American Pathologists (CAP, including the Personalized Healthcare Committee), the Association of Pathology Chairs (APC), and the American Society of Clinical Pathology (ASCP). He is past-president of the APC, and past-Editor-in-Chief of LABORATORY INVESTATION, an official journal of the US and Canadian Academy of Pathology (USCAP). He has served as Chair of the Council of Academic Societies of the Association of American Medical Colleges (AAMC), and on the AAMC Board of Directors.
He is author of over 240 peer-review papers, critical reviews, and book chapters, and senior editor of three books.